Researchers from Cedars Sinai Medical Center have developed a new technique that, if successful in humans, could replace traditional bone grafts for mending nonhealing limb fractures. The new method, which combines, microbubbles, ultrasound, and gene and stem cell therapies, effectively healed severely broken bones in a study in pigs.
In the U.S., approximately 100,000 bones fail to heal correctly, and more than 2 million bone grafts are performed around the globe each year. Bone grafts bridge gaps between the edges of a fracture that are too big for the bone to heal on its own. There are two kinds of bone grafts: autologous, where bone is taken from the patient’s own body, or allogeneic, where it is taken from a tissue bank.
However, both have drawbacks. Autografts, which are considered the current gold standard, are not always available in the volume required, may cause prolonged pain because of the second surgical site, and entail risk of infection and more time spent in the hospital, Gadi Pelled, Ph.D., DMD, assistant professor of surgery at Cedars-Sinai and the study’s co-senior author explained in an interview with Bioscience Technology.
“Allografts are readily available from tissue banks, but have a low capacity to form new bone and integrate with the patient’s bones,” he said. “They also have the risk of immune rejection and disease transmission.”
Hence, there is a large unmet need in skeleton repair.
For the new study, researchers built a scaffold made of collagen, which is a protein the body uses to build bones. It was implanted at the site of the bone break in laboratory pigs for two weeks, during which it “attracted” stem cells from adjacent bone marrow to populate the injury site, principal investigator and co-senior author of the study Dan Gazit, Ph.D. DMD, co-director of the Skeletal Regeneration and Stem Cell Therapy program in the Department of Surgery and the Cedars-Sinai Board of Governors Regenerative Medicine Institute told Bioscience Technology.
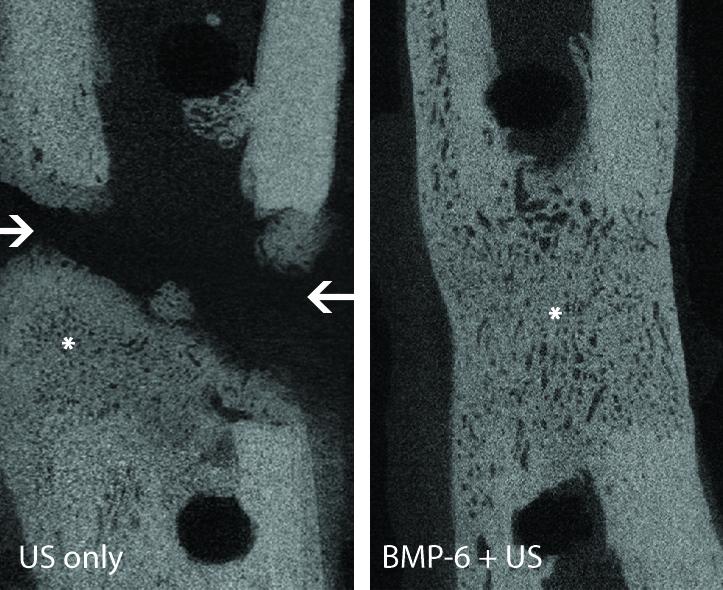
Next they injected microbubbles combined with genetic material called a plasmid, which is a short circular strand of DNA that codes for a protein known to induce new bone formation.
The DNA can’t enter the cells on its own, so ultrasound pulses and microbubbles facilitate the DNA entry across the cell membrane in a process called sonoporation, Gazit said.
“The ultrasound waves cause the microbubbles to oscillate, i.e. inflate and deflate,” Gazit explained. “The oscillation pulls gently on the cell membrane leading to the opening of tiny pores, which shut down very quickly, through which the DNA enters the cells.”
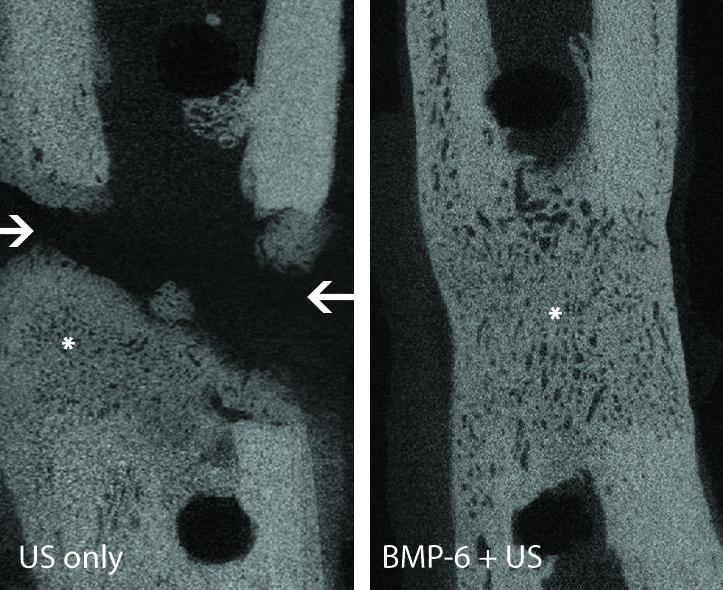
The technique promoted total bone healing and the leg fracture was mended in all of the animals within eight weeks after surgery. Expression of the introduced gene was undetectable after 10 days, and the bone grown in fracture had comparable strength to those produced by surgical bone grafts.
“This study is the first to demonstrate that ultrasound-mediated gene delivery to an animal’s own stem cells can effectively be used to treat nonhealing bone fractures,” Pelled said in a statement. “It addresses a major orthopedic unmet need and offers new possibilities for clinical translation.”
Zulma Gazit, Ph.D., co-director of the Skeletal Regeneration and Stem Cell Program in the Depart of Surgery and the Cedars-Sinai Board of Governors Regenerative Medicine Institute and co-author of the study, told Bioscience Technology that the team hopes the technique will translate into humans, and they are actively working towards that goal.
“If our technique is successful, bone grafts would not be needed for these types of bone loss cases,” she said. “In addition, we see great promise in the technology for the regeneration and repair of other tissues such as muscle ligaments and more. Wherever stem cells can be recruited and the regeneration factor (gene) is known, the system may be beneficial.”
Up next the team is planning to perform comprehensive research to address any concern about the safety of the technology prior to human trials.
“We are just at the beginning of a revolution in orthopedics,” Dan Gazit said in a statement. “We’re combining an engineering approach with a biological approach to advance regenerative engineering, which we believe is the future of medicine.”
The findings were detailed in a paper published May 17 in Science Translational Medicine.