A new method that leads to the formation of specialized tissue cells could improve the understanding of neurodegenerative diseases, inflammatory diseases and cancer.
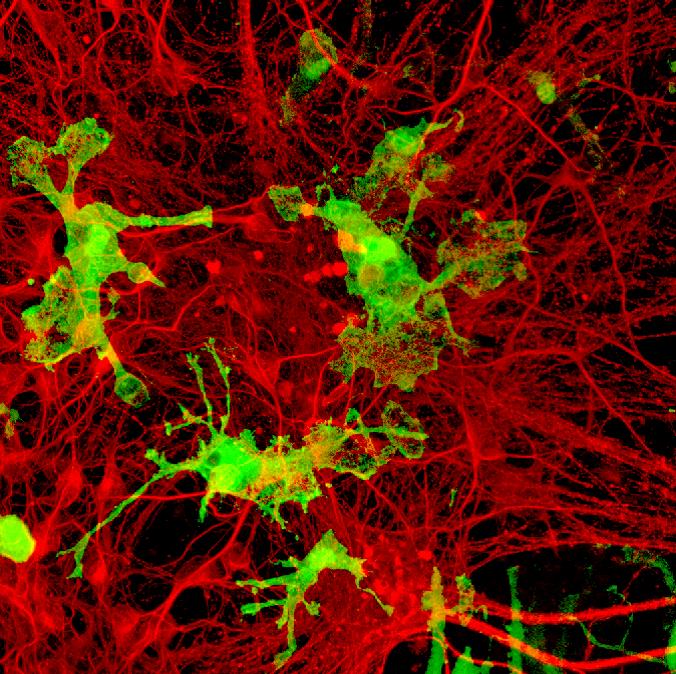
Credit : Kazuyuki Takata, Kyoto Pharmaceutical University
A new method that leads to the formation of specialized tissue cells could improve the understanding of neurodegenerative diseases, inflammatory diseases and cancer.
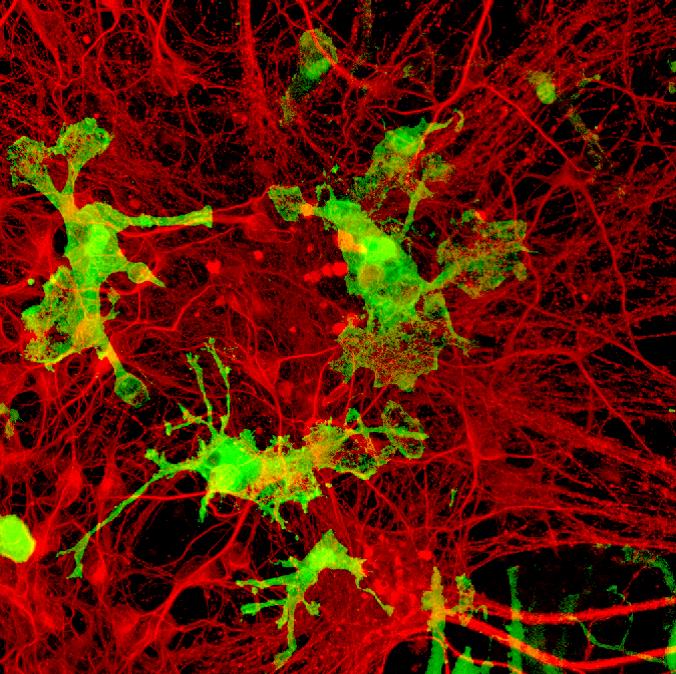
Macrophages are specialized cells involved in tissue development, repair and immunity. Macrophage communities in several types of tissues are derived from ‘yolk-sac precursor cells’, named primitive macrophages, planted there during early embryonic development. Isolating these precursor cells in sufficient numbers to study them has proven challenging.
An international team of researchers has found a breakthrough solution to this problem – developing a revolutionary protocol to induce the formation of primitive macrophages that are very similar to those derived from the yolk-sac. This will enable scientists to study primitive macrophage-derived cells in vivo, which is a critical step in advancing our understanding of various inflammatory diseases, cancer, and neurodegenerative diseases such as Alzheimer’s disease. The study was led by Dr Florent Ginhoux from the Singapore Immunology Network (SIgN), a research institute under Singapore’s Agency for Science, Technology and Research (A*STAR), together with Japan’s Kyoto Pharmaceutical University.
The team started with induced pluripotent stem cells (iPSCs): adult cells that are reprogrammed into an embryonic state, and are then directed to develop into any type of cell. By modifying currently available strategies, they were able to induce the formation of iPSC-derived primitive macrophages (iMacs) that are genetically, physically, and functionally similar to primitive yolk-sac-derived macrophages.
When they cultured iMacs with iPSC-derived neurons (nerve cells present in the brain), or transferred them into mouse brains, the iMacs developed into microglia, the resident macrophages of the central nervous system. The team also grafted the iMacs into the lungs of mutant mice with pulmonary alveolar proteinosis (PAP), a rare genetic lung disease. The iMacs matured into specialized lung macrophages that cured the disease, suggesting that iMacs could be used for cellular therapy treatment for various diseases.
The team also successfully developed iMacs from iPSCs derived from a patient with familial Mediterranean fever, a genetic disease characterized by recurrent fever and painful inflammation of the abdomen, chest and joints. The iMacs showed an excessive inflammatory response similar to that present in diseased individuals and can be used now as a cellular model to study the disease.
The new protocol, which has been patented by A*STAR, also yields a larger number of macrophages that more accurately represent their natural development in the body than previous methods.
The results demonstrate that the new protocol “could help to overcome the limitations placed on research into certain rare-disease entities by the lack of an adequate supply of disease-specific primary cells, and they might facilitate the development of novel therapeutic approaches, in particular for patients with PAP,” write the researchers in their recently-published study in the journal Immunity.