|
Getting your Trinity Audio player ready...
|
Stem Pharm Inc., a University of Wisconsin-Madison startup built on inventions related to the growth and control of stem cells, received a $290,000 grant Jan. 19 from the National Institutes of Health. The grant will support Stem Pharm’s continued development of sophisticated biological materials that can efficiently manufacture stem cells for medical use.
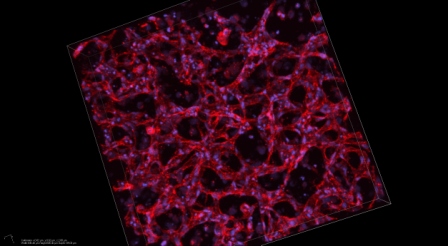
Stem Pharm’s custom materials support growing human cells to evaluate potential drugs or serve as replacement tissues for regenerative medicine. The company builds on research by founder William Murphy, a professor of biomedical engineering at UW-Madison.
Stem cells are highly versatile cells that have not yet specialized into adult cells, such as blood, muscle or nerve cells. Murphy focuses on finding better ways to direct stem cells to develop into adult cells and tissues.
This year, Stem Pharm plans to market systems that drug-makers can use to evaluate the safety and utility of drugs for treating blood vessels, liver and nerve tissue. Stem Pharm sells both biomaterial (which supports cell growth) and cells, depending on the market.
Human stem cells were greeted as miracle cures when they were discovered at the UW-Madison in 1998, but they are extremely difficult to work with. They are eager to develop into something else – but not necessarily whatever cell the scientists working with them intend.
Stem Pharm creates a synthetic, customizable Jell-O-like material that can support developing stem cells. “To manufacture a particular cell or tissue type,” Murphy says, “you often need to find a ‘Goldilocks’ environment” that balances characteristics like stiffness, adhesiveness, and responsiveness to chemicals secreted by the growing cells. “We rapidly identify the conditions needed to grow cells or tissues with the desired function. In some cases, the result is a cell with unique functions that would not exist without the material.”
Stem Pharm has licensed three patents related to the control of cell biomanufacturing through the Wisconsin Alumni Research Foundation. The patents are based on research in Murphy’s lab in the Wisconsin Institutes of Medical Research.
Over the last decade, studies there and elsewhere have shown the remarkable ability of materials to control virtually all aspects of cell function and tissue formation, Murphy says. “Our material serves as a concierge, to maintain the cells so they function as intended and remain where they are needed.” The same technology, he notes, can also apply to cells that are not derived from stem cells.
The company formed about 18 months ago. It has lab space in Fitchburg and two primary employees: Murphy, who works after his UW-Madison obligations are completed, and Connie Lebakken, president and chief operating officer. The company has several part-time employees and plans to expand if a current round of fund-raising succeeds.
Beyond making cells that enable testing of drugs, Stem Pharm is participating in the quest for cell therapy – replacement cells and tissues that might treat a wide variety of diseases and conditions. Already, Murphy notes, about 600 clinical trials are underway to test the regenerative ability of adult stem cells, such as the mesenchymal stem cells derived from bone marrow or fat.
These cell therapies must surmount certain “primary limitations,” Murphy says. “We need to be able to generate the cell or tissue efficiently and safely. They have to be manufactured at reasonable cost and they must function as intended at the desired location after being delivered into the body.”
The founding of the company, he says, “has come at a time when we realize that our custom biomaterials are very good at controlling cell behavior and we are zeroing in on applications that really require that control.”
Stem Pharm is interested in an eye condition known as age-related macular degeneration, a devastating condition that kills several cell types in the retina. As with all cell therapies, placing the new tissue is a significant challenge. “You don’t just want to get the cells to the target location, you want to place them in a way that allows them to organize and create the human tissue that was destroyed,” Murphy says. “We are exploring ways to generate the tissue outside the body and then implant it. We think the tissue can mature more precisely inside the body than outside.”
As Murphy has learned both in his lab and as co-director of the UW-Madison Stem Cell and Regenerative Medicine Center, stem cells teach a master class in the inherent intelligence of development. The process of specialization, honed through eons of evolution, created systems where developing cells almost magically respond to conditions by becoming something new.
That intelligence can be harnessed in for drug discovery, Murphy says. “We have discovered environments where cells that grow on a biomaterial with the right properties can self-assemble into their own three-dimensional tissue. That becomes exciting for drug discovery: the pharma just needs to seed the cells and walk away and they will assemble themselves, ready to be tested against thousands of potential drug compounds.”
The National Center For Advancing Translational Sciences of the National Institutes of Health funded some of this research under Award Number R43TR001904.