Researchers have identified a type of human leukocyte antigen (HLA) that is associated with the skin disease bullous pemphigoid (BP) in diabetic patients administered with DPP-4 inhibitory drugs.
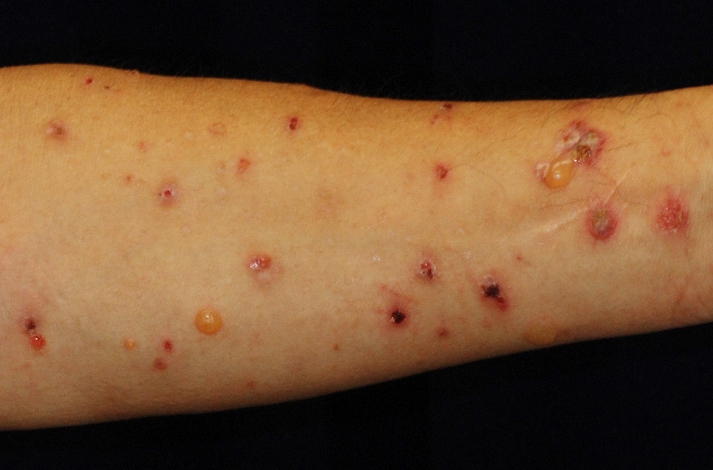
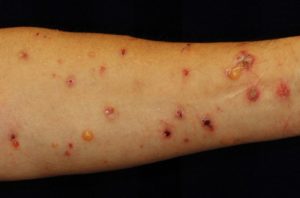 DPP-4 inhibitor (DPP-4i) is widely used to treat type 2 diabetes, but increased cases of bullous pemphigoid (BP) have been reported among patients taking the medicine. BP is the most common autoimmune blistering disorder, characterized by itchy reddening of the skin as well as tense blisters over the whole body. Afflicted patients ¬– mostly elderly – suffer from autoimmune attacks on a type of collagen in skin, making it hard to cure and compromising their quality of life. Previously, no risk factor triggering BP in diabetic patients administered with DPP-4i had been identified.
DPP-4 inhibitor (DPP-4i) is widely used to treat type 2 diabetes, but increased cases of bullous pemphigoid (BP) have been reported among patients taking the medicine. BP is the most common autoimmune blistering disorder, characterized by itchy reddening of the skin as well as tense blisters over the whole body. Afflicted patients ¬– mostly elderly – suffer from autoimmune attacks on a type of collagen in skin, making it hard to cure and compromising their quality of life. Previously, no risk factor triggering BP in diabetic patients administered with DPP-4i had been identified.
BP is classified into two types: inflammatory and noninflammatory, the latter of which is found more in diabetic patients administered with the drug. The research team, including Dr. Hideyuki Ujiie of Hokkaido University Hospital, examined 30 BP patients administered with DPP-4i, and investigated their symptoms and autoantibodies to group them as inflammatory or noninflammatory.
The researchers then analyzed human leukocyte antigen (HLA) genes of the 30 patients to identify their white blood cell type since HLA genes are known to be involved in various immune diseases. To compare, the team also analyzed the HLA of 72 BP patients who had not been administered with DPP-4i and 61 diabetic patients who were using the drug but not affected by BP. Their findings were compared with the HLA genes of 873 Japanese from the general population.
According to the results, 70 percent of the 30 BP patients administered with DPP-4i fell into the noninflammatory type with less reddening of the skin (erythema). HLA analyses found 86 percent of the noninflammatory BP patients administered with DPP-4i had an HLA gene called “HLA-DQB1*03:01.” The rate of having the HLA gene was much higher than was detected among the general population (18 percent) and non-BP type-2 diabetic patients administered with DPP-4i (31 percent). Meanwhile, 26 percent of BP patients who were not administered with the drug had the same HLA gene.
The findings show HLA-DQB1*03:01 is not linked to ordinary BP nor type-2 diabetes, but is closely associated with the development of BP among DPP-4i takers. “However, as the probability of patients exposed to DPP-4i to develop BP remains unclear, further research investigating a much larger number of cases is needed,” says Hideyuki Ujiie.
“Our results suggest people with HLA-DQB1*03:01 have a higher risk of developing BP when exposed to DPP-4i than those without the HLA gene. The gene could serve as a biomarker to help estimate the risk of developing BP when patients are administered with DPP-4i. The mechanism that connects the HLA gene and BP needs to be addressed to help prevent the development of the disease,” Ujiie added.