Propylene is a colorless, flammable hydrocarbon gas that is an important raw material for the production of a variety of petrochemicals. Due to increasing demand and limited global supply, there is a strong need to develop new, efficient technologies for its production.
Researchers at Hokkaido University have developed an innovative catalyst for the production of propylene that is highly active, selective, stable, and utilizes carbon dioxide (CO2) efficiently. Their findings were reported in the journal Nature Catalysis.
One promising technique for producing propylene is a chemical reaction, called oxidative dehydrogenation, that uses CO2 to convert propane gas into propylene by removing hydrogen. However, existing catalysts used to speed up this chemical reaction aren’t very efficient.
“The challenge is to develop a catalyst that will activate both reactants – propane, and CO2 – without unwanted side reactions. It also needs to be stable and reusable in the long term,” explains Hokkaido University molecular engineer, Shinya Furukawa.
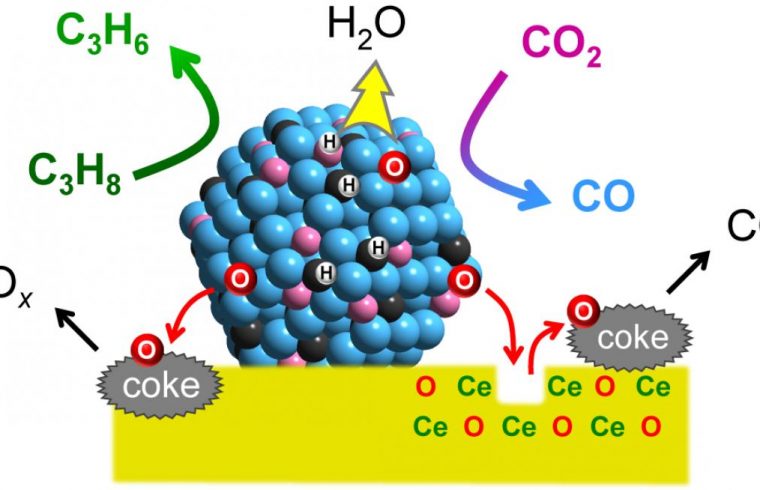
To achieve this, Furukawa and his colleagues developed a catalyst made from three different metals (platinum, cobalt, and indium), each chosen for its specific properties. Platinum was selected as the main active metal because of its ability to break chemical bonds between carbon and hydrogen, enabling the dehydrogenation reaction.
Cobalt accelerates CO2 capture and activation, while indium enhances the catalyst’s selectivity. The metals were fixed to support made from cerium oxide, a compound commonly used in car catalytic converters.
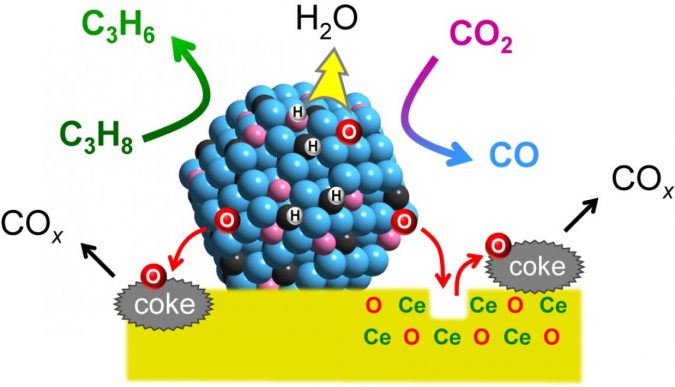
The researchers tested the catalyst’s activity at 550°C and compared the results with existing catalysts. They also performed a mechanistic study to understand the functions of the different components and found the catalyst links the propylene-forming reaction to the deoxygenation of CO2, and ensures the catalytic activity is specific to propane; water, and carbon oxides are formed as byproducts.
Further, they found that the catalyst increased the reaction rate approximately fivefold compared to the typical values reported from other systems. The reaction produced a higher ratio of propylene and utilized more CO2 at 550°C compared to previous catalysts. The catalyst also showed good long-term stability and reusability.
“To date, no other catalyst has been shown to simultaneously exhibit high catalytic activity, selectivity, stability, and CO2 utilization efficiency. Our multifunctional material meets all these requirements,” says Furukawa.
This study provides new insights into the design of highly efficient catalysts for petrochemical production and has potential benefits for carbon recycling and greenhouse gas reduction.