Researchers at Tohoku University have unearthed a means to stabilize lithium or sodium depositions in rechargeable batteries, helping keep their metallic structure intact. The discovery prevents potential battery degradation and short-circuiting and paves the way for higher energy-density metal-anode batteries.
Scientists are ever-seeking to develop safer, higher-capacity, and faster-charging rechargeable batteries to meet our energy needs sustainably. Metal anodes show the highest promise to achieve that goal. Yet the use of alkali metals poses several problems.
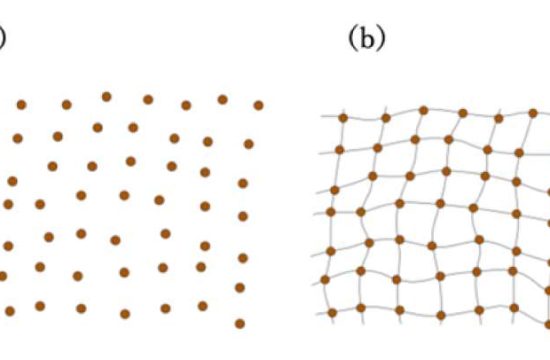
In a rechargeable battery, ions pass from the cathode to the anode when charging, and in the opposite direction when generating power. Repeated deposition and dissolution of metal deforms the structures of lithium and sodium. Additionally, fluctuations in diffusion and electric fields in the electrolytes close to the electrode surface lead to the formation of needle-like microstructures called dendrites. The dendrites are weakly bonded and peel away from the electrodes, resulting in short circuiting and decreases in cycle capacity.
To solve this problem, a research team led by Hongyi Li and Tetsu Ichitsubo from Tohoku University’s Institute for Materials Research added multivalent cations, such as calcium ions, that altered and strengthened the solvation structure of lithium or sodium ions in the electrolyte.
“Our modified structure moderates the reduction of lithium or sodium ions on the electrode surface and enables a stable diffusion and electric field,” said Dr. Li. The stabilized ions, in turn, preserve the structure of the electrodeposited metals.
Details of their research were published in the journal Cell Reports Physical Science on May 20, 2022.
For their next steps, Li and Ichitsubo are hoping to improve the metal anodes’ interfacial design to further enhance the cycle life and power density of the batteries.