Researchers have created intermetallic alloy nanoparticles of palladium and zinc with an alternating arrangement of zinc and palladium atoms.
The intermetallic alloy is more corrosion-resistant than substitutional alloys while retaining the electrocatalytic properties of both metals, which could be useful for developing new non-precious metal electrocatalysts.
Palladium—a precious metal—is attracting attention as a fuel cell electrocatalyst, which requires metals with high electrocatalytic activity. Because of the high cost of palladium, creating a palladium-zinc alloy should improve catalytic activity while reducing costs.
In addition, corrosion resistance is important for potential electrocatalyst materials because catalytic reactions use extremely corrosive alkaline aqueous solutions that degrade metal electrocatalysts and lower their efficiency over time.
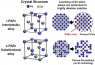
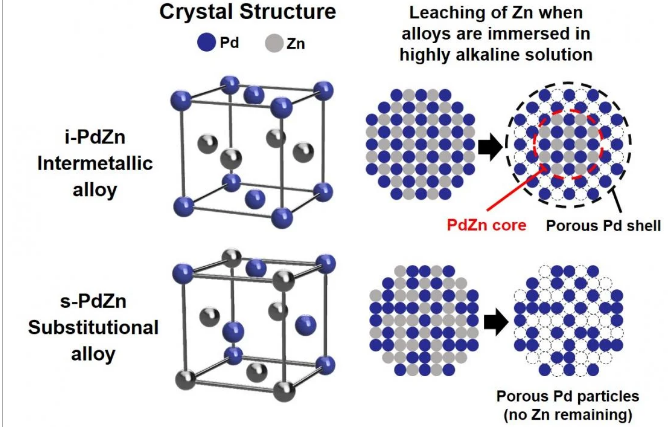
A research group led by Professor Hiroshi Inoue, Associate Professor Eiji Higuchi, and Associate Professor Masanobu Chiku of the Graduate School of Engineering, Osaka Metropolitan University, has found a promising intermetallic alloy of palladium and zinc (i-PdZn). The intermetallic alloy has a precisely arranged atomic structure consisting of equal amounts of alternating palladium and zinc atoms. This makes the i-PdZn highly resistant to corrosion in alkaline aqueous solutions.
Metal alloys can be substitutional or intermetallic. In a substitutional palladium-zinc alloy, zinc atoms replace half of the palladium atoms at random, whereas palladium and zinc atoms in the new intermetallic alloy i-PdZn are arranged in an alternating fashion so that each zinc atom is surrounded by palladium atoms and vice versa.
When both the i-PdZn and the substitutional palladium-zinc alloy were immersed in an aqueous alkaline solution, the researchers found that most of the zinc atoms leached out of the substitutional alloy within a few minutes.
However, the regular arrangement of palladium and zinc atoms in the i-PdZn effectively prevented the zinc atoms in the middle of the alloy from leaching out. This created a protective skeletal palladium shell around the outside of the alloy, giving the i-PdZn a much higher corrosion resistance.
Furthermore, when used as an electrocatalyst to generate, the i-PdZn exhibited a peak ethanol oxidation activity approximately 5.1 times as high as that of pure palladium. This was due to both the electronic properties of the alloy’s core, and the larger surface area created by the skeletal palladium shell in the aqueous alkaline solution.
“The unique properties of intermetallic alloys that make them good catalysts were previously known, but now we have shown that the dissolution of zinc is greatly suppressed in our intermetallic palladium-zinc alloy, which makes it much more corrosion-resistant than the substitutional alloy,” Professor Inoue concluded. “We believe that these results may provide clues for the development of non-precious metal electrocatalysts, for which corrosion resistance is an issue.”
The research results were published online on July 23, 2022, in Research on Chemical Intermediates, a journal published by Springer Nature.