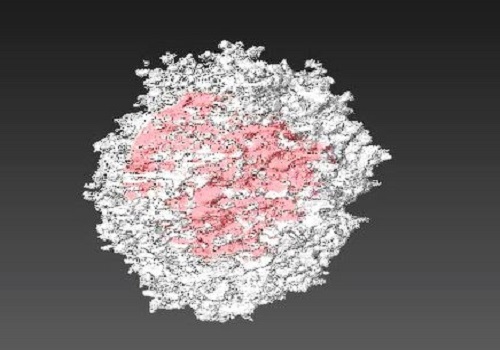
Nonsurfactant polymers template produces a highly porous, three-dimensional inorganic crystal to enhance catalysis and separation.
Reproduced with permission from reference 1© 2016 WILEY-VCH
High-performance porous materials needed for the selective catalysis and the separation of large compounds have been developed by researchers at King Abdullah University of Science and Technology (KAUST), Saudi Arabia.
Zeolite is one such porous material that combines aluminum, silicon and oxygen into a crystalline network of molecular-sized cavities and channels. Its pores typically measure less than one nanometer because their synthesis uses small cations as templates. The tiny size and network configuration result in extensive surface areas as well as size and shape selectivity, which is a boon for catalysis and separation. On the other hand, this size also is limiting, as there is slow diffusion and low catalytic activity in cases where bulky molecules are involved.
These size-related problems may be circumvented by hierarchical zeolites, which have micropores and larger pores wider than a few nanometers. While micropores provide catalytic activity and selectivity, the mesopores allow bulky molecules to rapidly diffuse through zeolites.
[pullquote]Han’s team has now developed a method that produces these 3-D hierarchical zeolites using nonsurfactant polymers as templates.[/pullquote]
To date, the production of hierarchical zeolites has relied on surfactants as templates. These molecules, which simultaneously bear water-loving and water-repelling functional groups at their extremities, form layers alternating with zeolite sheets to give two-dimensional (2-D) structures.
“From an application perspective, it’s better to have three-dimensional (3-D) structures than 2-D sheets,” said Yu Han from KAUST Advanced Membranes & Porous Materials Center (AMPMC).
Han’s team has now developed a method that produces these 3-D hierarchical zeolites using nonsurfactant polymers as templates. The zeolites have highly crystalline structures with open and interconnected mesopores. In addition, the polymers also generate unique composites in which mesoporous zeolites are able to coat zeolite seeds.
According to Han, sheets arise from strong intermolecular interactions between surfactant molecules. The team chose nonsurfactant polymers because “we hypothesized that a 3-D structure would be produced if we minimized these interactions,” he said. The polymers play a dual role: they assist mesopore production, while their many positively charged ammonium functional groups direct the zeolite crystallization.
The researchers compared the catalytic performance of the hierarchical zeolites to that of bulk analogues for two reactions. When methanol was converted into aromatic hydrocarbons, in which the buildup of coke by-product deactivates catalysts, the zeolites outperformed their analogues. They maintained their activity longer and showed a higher conversion capacity and rate constant. Moreover, in the catalytic cracking of canola oil, which involves bulky reactants, they gave the highest conversion yields.
“We are now exploring the full potential of these hierarchical zeolites for catalysis and separation applications with colleagues from KAUST Catalysis Center and the University’s AMPMC,” Han said.