By Ramesh Marasini, Ph.D.
Within every human body, a troop of soldiers stand at attention, dutifully prepared to seek and destroy identified harmful invaders (diseased cells and foreign objects). These service-minded soldiers, also known as immune cells, inspired the researchers to make novel immune cell-like material to help doctors improve cancer diagnosis and treatment.
For years, researchers have tried to balance treatments that target drugs to cancerous cells and the severe side effects of existing anticancer drugs, but the perfect balance has been a challenge.
Scientists from Kansas State University have developed a new methodology for treatment mimicking the natural process of immune cells- a biomimetic approach published in the journal Advanced Functional Materials. This technology may guide treatments to the disease site by the use of an external magnet helping early diagnosis, minimize severe side effects of existing chemotherapeutics, and accelerate the patient’s healing process.
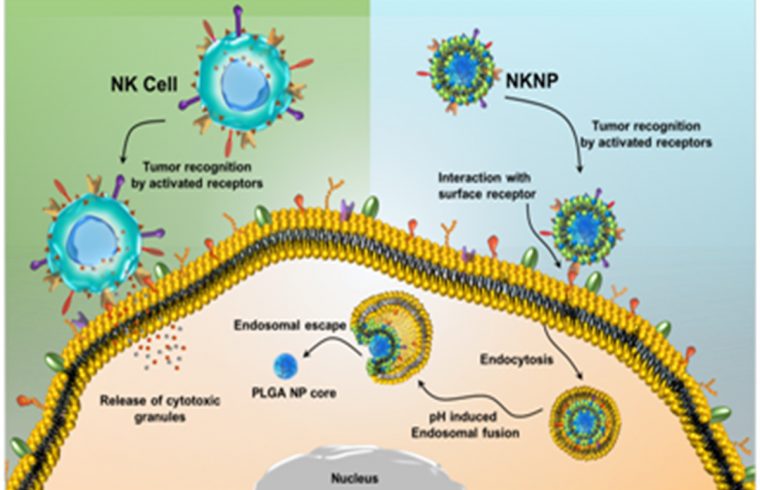
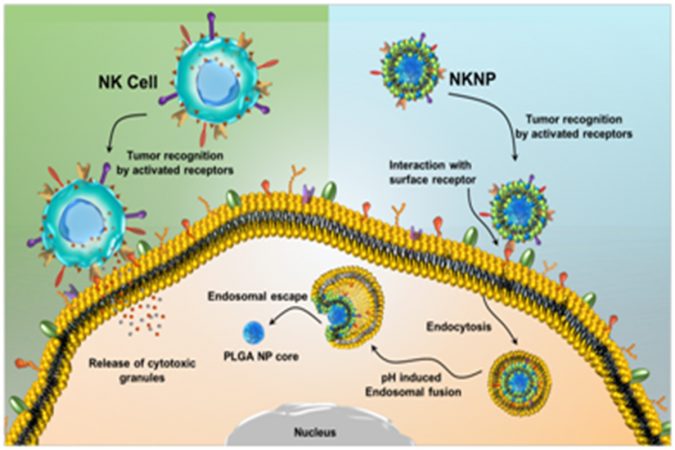
This method uses a bio-nanomaterial (one billionth of a meter-sized material) taking inspiration from the body innate immune system’s natural killer (NK) cells. NK cells are large granular lymphocytes that belong to the inherent immune system, and their major function is to provide host defense against microbial infections and tumors by immune surveillance.
Since the population of NK cells in the human body is very low, it is very difficult to use them in large amounts for cell-based cancer therapy. To address, scientists have engineered NK cells with synthetic components where both could collaborate acting like a NK cell that naturally seeks and destroys cancer. This NK cell membrane discretely camouflaged biomimicry actually minimizes the absorption of cancer-related drugs- chemo drugs and contrast drugs used for imaging- into vital organs where they have the potential of becoming toxic.
For example, the repeated and high dose of gadolinium, a heavy metal used to light up nearly 40% magnetic resonance images for the diagnosis, containing contrast drugs has been found to gadolinium deposition in the brain, kidney, and bones. Recently, these findings prompted the authorities in the European Union to pull some of the clinically approved gadolinium contrast drugs. However, all approved gadolinium contrast drugs are used in the United State with a labeled warning sign of gadolinium retention and suspected toxicities.
To address the alarming toxicity issue and lack of specificity of current drugs used in cancer diagnosis and treatment, scientists have designed a biomimetic delivery system using both- anticancer and contrast drugs- simultaneously that has shown significant tumor reduction and minimize high dose gadolinium exposure limiting potential negative side effects. The developed method for tumor visualization performed three hundred percent better than Magnivist®, a clinical contrast drug. Notably, the specimens received two-fold lower doses of gadolinium.
This technology comparable to the “quasi-nano-robot” gave promising results in tumor mapping and treatment in the mouse model. These ‘quasi-robots’ are biological soldiers engineered with biodegradable polymers and distinct magnetic features to show response with an external magnet used in magnetic resonance imaging allowing them to be strategically guided inside the body. This could help the radiologist to diagnose cancer more accurately at the early stage of development and offer the opportunity for treatment planning.
Researchers are also applying these findings for the diagnosis of secondary tumors that could prevent cancer cells from migration throughout the body.
Considering these ‘remote-controlled tiny quasi-robots’ have the ability to release life-saving drugs to difficult-to-reach areas and are able to sense distinct bodily changes linked with the onset of illness, cell-based hybrid nano-robots could allow doctors to diagnose disease and deliver drugs with extreme precision.