A breakthrough in material science could help deliver a new generation of affordable batteries, scientists say.
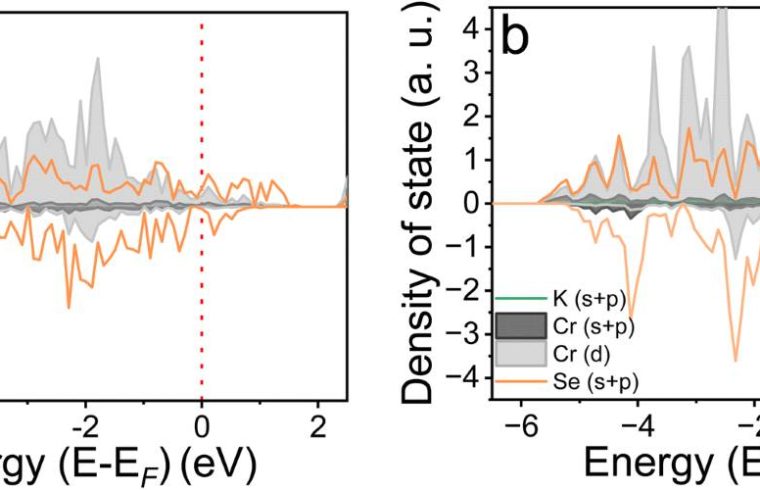
An international team of researchers led by chemists from the University of Glasgow and battery testing experts at Helmholtz Institute Ulm have implemented a material made from chromium and selenium in a potassium-ion battery.
The discovery brings batteries a step closer to becoming a viable alternative to lithium-ion systems, thanks to the abundant availability of potassium and its advantageous material properties, such as rapid charging.
Experts believe that the batteries could be cheaper and easier to manufacture than lithium-ion batteries, opening up applications including the storage of electricity generated from renewable sources.
Dr. Alexey Ganin, of the University of Glasgow’s School of Chemistry, is the paper’s lead author and the Head of the Glasgow ElectroChemistry on Solids (GECOS) group. He said: “Lithium-ion batteries have become widely adopted in devices from smartphones to electric cars in recent years, and are capable of excellent performance, but lithium is a relatively rare, and therefore strategically important, element.
“Potassium is a much more abundant material, and potassium-ion batteries have a lot of potential as an alternative method of storing and delivering large amounts of electricity. Adopting potassium-ion batteries for stationary storage purposes could help free up lithium resources for future energy-intensive mobile applications.”
Some of the best-performing current designs for potassium-ion batteries use cathodes made from a material called Prussian White. However, their design is complicated by the need to mix Prussian White with carbon to boost its conductivity to deliver the best results.
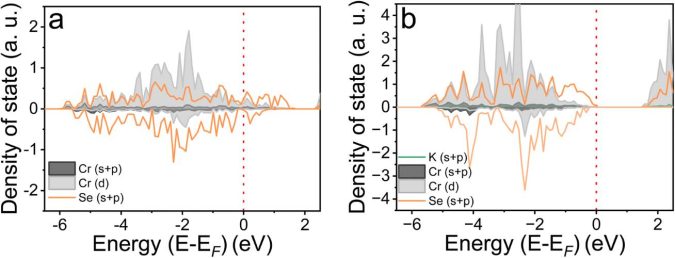
In a paper published in the Journal of Materials Chemistry A, the researchers show how their naturally conductive chromium selenide cathode achieves high performance with less than 10 % carbon. Their prototype has a capacity of 125 milliamp-hours per gram, very close to its maximum theoretical capacity of 127 milliamp-hours per gram.
The layered nature of the material allows potassium ions to travel more easily between the layers during charge and discharge. This allows the battery to maintain 85% of its capacity in lab conditions even when charged and discharged at high speed.
The next step for the team is further research to identify an electrolyte that will help deliver improved performance in future refinements of the battery’s design.
Dr Ganin added: “These are promising results, but we believe the performance of the battery could be boosted further with the right electrolyte. Designer lithium-ion battery electrolytes can be bought off the shelf, but further work is required to refine the performance of electrolytes for potassium-ion batteries. We’re keen to partner with robotics experts who can help us test the thousands of potential chemical combinations to find the best possible candidate for use in our battery.”
Researchers from Ulm University, the Karlsruhe Institute of Technology, the University of Kent, the Catalan Institute of Nanoscience and Nanotechnology, and ICREA also contributed to the paper.
The team’s paper, titled ‘Reversible K-Ion Intercalation in CrSe2 Cathodes for Potassium-Ion Batteries: Combined Operando PXRD and DFT studies’, was published in the Journal of Materials Chemistry A.