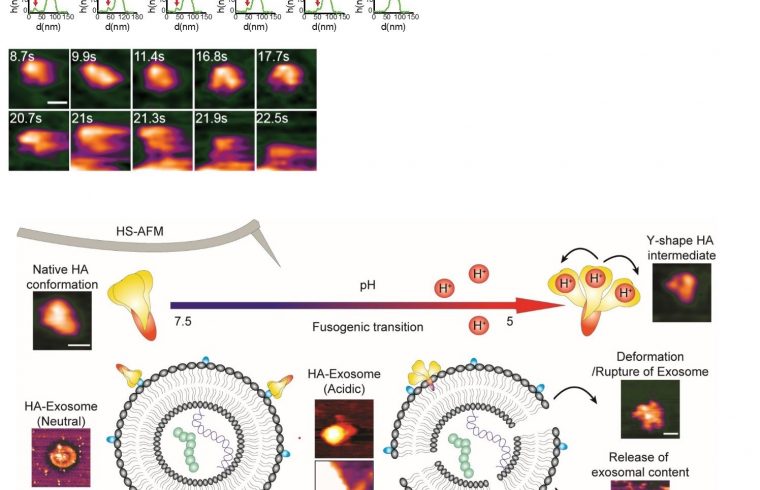
The real-time visualization of influenza A hemagglutinin (HA) has enhanced the understanding of fusogenic transition of HA and its interactions with host endosomes.
A high-speed atomic force microscopy study on a biological event that happens during flu virus enters infects its host cell. This study was published in Nano Letters, a monthly peer-reviewed scientific journal.
Unlike living organisms, to avoid extinction, viruses need to hijack living host machineries to generate new viruses. The devastating respiratory virus, influenza A virus, utilize its hemagglutinin (HA) proteins to search for suitable host cells.
Generally, HA has two important functions: selection of host cell and viral entry. Upon attaching to host cells, Influenza A virus are brought into host cells via endocytosis. A lipid bilayer cargo, known as endosome, carries influenza A virus from cell membrane into cytoplasm of host cell. Although the environment inside endosome is acidic, influenza A virus remains alive.
More strikingly, HA undergoes structural change to mediate viral membrane to fuse with host endosomal membrane to form a hole in order to release viral components. Generation of this fusion event is elaborated as fusogenic, and hence structural changes of HA needed for this event is called as fusogenic transition.
The mechanism of this event has been kept in Pandora’s Box for decades despite extensive studies have been done to reveal its mystery.
Now, Keesiang Lim and Richard Wong from Kanazawa University and colleagues have studied the molecular dynamic of HA using high-speed atomic force microscopy, a technique enabling real-time visualization of molecules on the nanoscale.
The researchers were not only able to record the fusogenic transition of HA, but also observe its interaction with exosomes (a lipid bilayer cargo similar to endosome released by cells to outside environment).
During Research
The scientists initially observed the native conformation of HA under neutral physiological buffer, a condition that resembles to a neutral condition in host cell (a pH of 7.6). In this condition, HA was appeared as an ellipsoid, which is in agreement with findings generated by other tools such as X-ray crystallography and cryo-electron microscopy.
Wong and colleagues have successfully recorded the fusogenic transition, which happening when HA was exposed to an acidic environment. Their HS-AFM results illustrated a transition of HA from an ellipsoid to a Y-shape together with declination of height and circularity/roundness of HA over time.
The researchers reassure the conformational change happens because a particular subunit of HA became easily to be digested by trypsin after the transition.
What is Influenza A hemagglutinin?
Influenza A hemagglutinin (HA) is a protein residing on the surface of influenza A virus (the culprit that causes ‘the flu’ or influenza), playing a key role in viral infectivity. HA’s functions include attaching influenza A virus to target cells and viral entry.