Osaka, Japan – For centuries, humans have made use of glass in their art, tools, and technology. Despite the ubiquity of this material, however, many of its microscopic properties are not well understood, and it continues to defy conventional physical description.
Enter Koun Shirai of The University of Osaka. In an article published in Foundations, Shirai bridges conventional physical theory and the study of nonequilibrium materials to provide a robust description of the thermodynamics of glasses.
Most materials exist in an equilibrium state, meaning that the forces and torques on the material’s atoms are all balanced. Glasses, however, are a famous exception: they are amorphous solid materials whose atoms are always rearranging, albeit very slowly, toward an equilibrium state but do not exist in equilibrium.
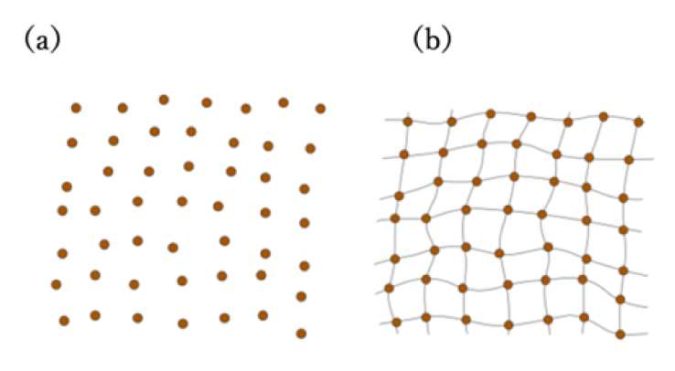
“If you compare the atomic structures of glasses and crystals, they are actually very different,” says Shirai. “Crystals have atoms that are arranged in neat lattices, whereas glasses have atoms that are more disorganized, similar to a liquid. Thus, one traditionally thinks of glasses as out-of-equilibrium liquids that flow very, very slowly.”
This lack of an equilibrium state has posed a major challenge for generations of physicists. Since glasses are technically not in equilibrium, the standard laws of thermodynamics do not apply to them, making them difficult to analyze.
“In thermodynamics, systems are usually characterized in terms of variables called order parameters,” explains Shirai. “Order parameters can be used to describe what state a material is in or how close it is to changing states. However, the paradox is that glasses are inherently disordered, so how do we meaningfully define such parameters for these materials?”
To address this question, Shirai had to redefine what “equilibrium” means: in his framework, a material is in equilibrium if energy cannot be extracted from it without impacting its surroundings. From this perspective, glasses are actually in equilibrium, and the tools of thermodynamics can be made to apply with some modification.
“It can be shown that the order parameters are nothing more than time-averaged positions of the atoms,” says Shirai. “In this way, we can unify the thermodynamic description of glasses with that of other solid materials such as crystals.”
Shirai believes that this new formulation will help illuminate the physics of other materials that are typically classified as being out of equilibrium, such as biological systems. Thanks to his research, it may not be long before we have a full thermodynamic description of other nonperiodic and complex materials.