|
Getting your Trinity Audio player ready...
|
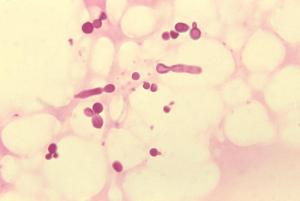
Full genomic sequencing of all 14 species of the Malassezia genus opens up possibility of new treatments for microbially-mediated skin diseases.

Copyright : Wikimedia
Singapore – An international team of scientists, led by researchers from A*STAR’s Genome Institute of Singapore (GIS), Institute of Medical Biology (IMB), and Bioinformatics Institute (BII), and P&G, have completed the first comprehensive genomic and biologic study of all species of Malassezia, one of the top skin disease-causing microbes. The breakthrough study identified multiple potential targets for treating diseases such as seborrheic dermatitis, eczema and dandruff, all of which can be caused by Malassezia. Malassezia is also associated with skin cancer, the 6th most common cancer in males and the 7th in females in Singapore[1]. These findings improve our understanding of the human skin microbiome, with implications for dermatology and immunology. The study was published in the November issue of PLOS Genetics.
Malassezia is a type of yeast found on the skin of all birds and warm-blooded mammals, including humans. Often, Malassezia simply forms part of our normal skin flora, but for unknown reasons it sometimes causes disease. In particular, two species of Malassezia, M. restricta and M. globosa, are present on all human scalps and are responsible for common dandruff and seborrheic dermatitis[2]. Dandruff occurs when Malassezia feeds off fatty external lipids secreted naturally on the scalp, and the partially digested lipids lead to irritation. The link between dandruff and the two species was first discovered, and their genomes fully sequenced, by Dr Thomas Dawson and his team at P&G in 2007, which also developed subsequent hair care technologies to target them. However, much remained unknown about Malassezia.
[pullquote]Scientists are increasingly turning to the human microbiome and the microorganisms living on humans for greater insight on human health.[/pullquote]
By sequencing the genomes of all known Malassezia, (including multiple strains of those most common on human skin), the team identified hundreds of features explaining how the fungus may be able to thrive on human skin. The dependence of all Malassezia species on lipids for survival was also established, and the idea that they are sexually active remains supported. Through this knowledge, scientists can start finding ways to control their activity on human skin, and work towards the restoration of healthy skin.
Importantly, a gene unique to Malassezia and in no other related fungi, was also found. This gene is potentially the one which first allowed Malassezia to switch from living only on plants to being able to live on animals, birds and humans. In other words, by targeting this gene, we may be able to eliminate Malassezia on human skin, or weaken its growth and survival significantly. This discovery thus lays the groundwork for future work to develop therapeutics targeting the gene for a range of skin diseases.
The study is particularly relevant to Singapore and Southeast Asia, given that the hot and humid climate provides a perfect environment for fungi to thrive. In fact, Singapore has the highest reported incidence of fungal-mediated skin disease in the world[3], with about one in five people suffering from atopic dermatitis, or eczema[4]. Therapeutics and further research addressing Malassezia would therefore have widespread applications in the region.
Furthermore, scientists are increasingly turning to the human microbiome and the microorganisms living on humans for greater insight on human health. For example, the US National Institutes of Health (NIH) launched the Human Microbiome Project (HMP) in 2008 with a budget of US$115 million over five years[5]. Malassezia plays a dominant role in the human microbiome and knowing more about these fungi substantially improves our overall understanding. In particular, a database with the sequenced Malassezia genomes has been made public, allowing researchers worldwide to learn more about what was previously considered ‘dark matter’ on the skin.
Dr Thomas Dawson Jr., Senior Principal Investigator at A*STAR’s Institute of Medical Biology (IMB), principal author of the paper, and who previously led studies on Malassezia while at P&G, stated, “This new information will allow us to better understand healthy versus unhealthy skin and hopefully learn to modulate the skin microbiome so as to transform unhealthy into perfect, healthy skin.”
Dr Niranjan Nagarajan, Principal Investigator at A*STAR’s Genome Institute of Singapore (GIS) and co-author of the paper, said, “This study provides a comprehensive genetic resource for further investigations into Malassezia biology and its life on human skin. Particularly exciting was the identification of several genes horizontally acquired from bacteria that play an important role in making Malassezia unique.”
Dr Benjamin Seet, Executive Director of A*STAR’s Biomedical Research Council (BMRC), commented, “This study helps us understand how a microscopic organism that lives on the skin can give rise to a common disease like eczema that affects one in every five Singaporeans, as well as to serious conditions like skin cancer. Our partnership with P&G opens doors to important research that will benefit patients suffering from these conditions in Singapore, and which will otherwise not be conducted here.”
Dr Jim Schwartz, P&G Research Fellow, observed, “Malassezia are at the centre of the incurable yet treatable conditions of dandruff and seborrheic dermatitis. The continued investment in understanding the fundamental nature of these organisms allows a more in-depth appreciation of how and why they trigger the annoying symptoms that reduce quality of life for those responsive to their metabolic activities. Being at the forefront of research in this field assures our therapeutic product technology will likewise represent state of the art efficacy now and in the future.”