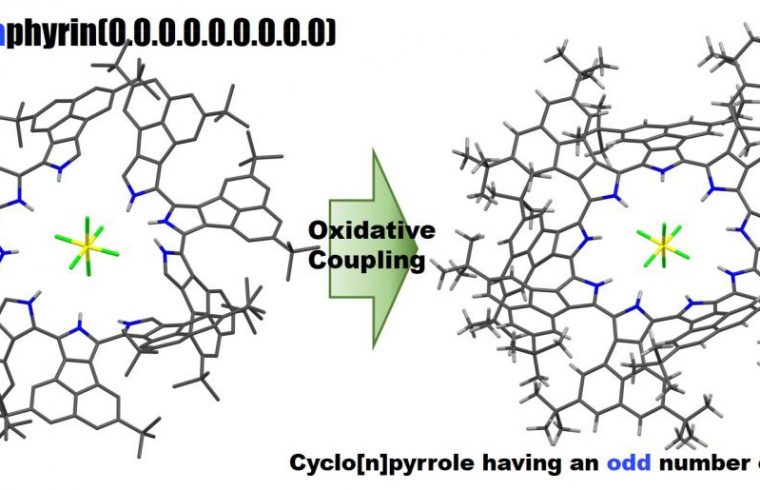
A ring-expanded porphyrin with no meso-bridges comprised of an odd number of pyrroles was successfully synthesized via the oxidative coupling of the corresponding terpyrrole.
Porphyrins, which are well-known natural porphyrin molecules, e.g. heme and chlorophyll, are attractive for use in practical materials because of the easy optimization of their optical and physical properties by conjugation expansion and functionalization.
This porphyrin showed a 34pi aromatic character and an intense absorption at the near-infrared region. They analyzed the optical and electronic structures using magnetic circular dichroism spectroscopy and time-dependent density functional theory calculations.
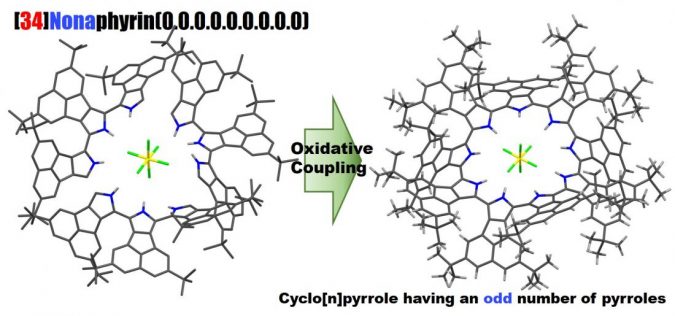
Profs. Okujima and Uno at Ehime University, in collaboration with Prof. Kobayashi at Shinshu University, reported the selective synthesis, the molecular structure, optical properties and electronic structure of cyclo[9]pyrrole, a ring-expanded porphyrin consisting of directly connected pyrrole rings.
In 2002, Sessler reported the first synthesis of cyclo[n]pyrrole (n: the number of pyrrole rings). Peripheral alkyl-substituted cyclo[8]pyrroles were obtained via an oxidative coupling of 2,2’-bipyrrole, and showed an intense L band at ca. 1,100 nm.
Researchers successfully synthesized a good yield of cyclo[9]pyrroles via the oxidative coupling of terpyrrole. A relatively distorted structure with a C2-like symmetry was clarified by NMR and X-ray diffraction analyses.
Intense absorption was observed at ca. 1,740 nm. “We analyzed the optical and electronic structures using magnetic circular dichroism spectroscopy and time-dependent density functional theory calculations,” in a statement.
They were compared cyclo[8], [9], and [10]pyrroles, showed the electronic structures don’t significantly depend on the number of pyrrole.
Findings were published on April 15, 2021 in Organic Letters.