A collaborative work by a group of researchers from Malaysia and Australia have lead to the discovery of a significant novel finding on the ultrasound-induced formation of a new type of super viscoelastic micelles.

“Micelle is a very beautiful ‘feministic‘ creation. With the simultaneous existence of hydrophilic and hydrophobic properties, it has the ability to form a wide range of structures which are beneficial to our daily lives. Micelle may obediently follow the aggregational ‘commands’ of stimuli (e.g. temperature, pH etc) and remain with great stability as the desired structure. With the right condition, it can also flexibly reform as how the stimuli order it to be.”
[pullquote]Household products such as soap, beauty creams, lotions, detergents, cleaning liquids etc contain surfactants in the form of micelles.[/pullquote]
“In the materials world, when sound (ultrasound, US) meets micelle(s), the law of nature guides the events. He (US) takes her (micelle) hands to the dancing floor (reaction medium) and with gracious steps to the melody (physical forces generated by US), transforms her into a beautiful structure.”
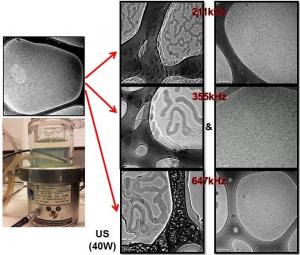
Household products such as soap, beauty creams, lotions, detergents, cleaning liquids etc contain surfactants in the form of micelles. Micelles possess unique ability to exhibit different physicochemical properties owing to their dynamic and reversible structural transformation, controllable by different stimuli. Viscoelasticity of a micelle system is mainly governed by the length of the micelle structure formed in each system, i.e. wormlike micelle possess greater viscoelasticity than rodlike micelle. However, a particular micelle system possesses a growth limit where branching or deformation may occur upon further stimulus action.
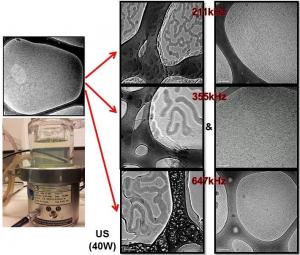
A collaborative work by a group of researchers from Malaysia and Australia have lead to the discovery of a significant novel finding on the ultrasound-induced formation of a new type of super viscoelastic micelles using a well-known micelle system consisting of cetyltrimethylammonium bromide and sodium salicylate. The viscoelastic changes of micelles occurred due to the variation of their structural aggregation caused by the ultrasound ‘command’ (e.g. frequency, power, reactor) to the system. Amazingly, the micelles responded to ultrasound by simultaneously forming a new structure with an increase in viscoelasticity (long threadlike micelle) and another structure with a decrease in viscoelasticity (tubular micelle/vesicle), simultaneously upon sonication. Such ultrasound responsive micelle may be utilized in systems where a very viscoelastic surfactant is desired as well as when both high and low viscoelastic properties are simultaneously needed. The mechanism for such transformation has also been proposed for on a wide range of sonication experimentation and the micellar structural responses.