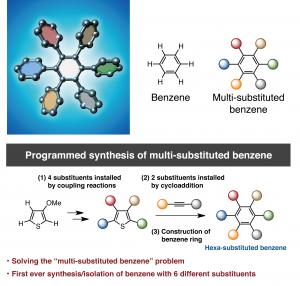
 Chemists at the Institute of Transformative Bio-Molecules (ITbM), Nagoya University and the JST-ERATO Project have developed a new method to accomplish the programmed synthesis of benzene derivatives with five or six different functional groups that enables access to novel functional organic materials that could not have been reached before.
Chemists at the Institute of Transformative Bio-Molecules (ITbM), Nagoya University and the JST-ERATO Project have developed a new method to accomplish the programmed synthesis of benzene derivatives with five or six different functional groups that enables access to novel functional organic materials that could not have been reached before.
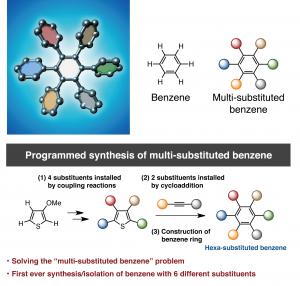
Nagoya, Japan – Professor Kenichiro Itami, Junichiro Yamaguchi, Yasutomo Segawa and Shin Suzuki at the Institute of Transformative Bio-Molecules (ITbM), Nagoya University and the JST-ERATO Itami Molecular Nanocarbon Project have developed a new synthetic methodology to achieve the first programmed synthesis, isolation and characterization of a multi-substituted benzene derivative with five or six different functional groups. Benzene is one of the most common structures in pharmaceuticals and multi-substituted benzene derivatives are found in many organic electronic devices. Despite being highly useful, multi-substituted benzene derivatives are rather difficult to synthesize due to the lack of selective methods to install different substituents at the desired positions. Driven by the high necessity to access such materials, Itami’s group has devised a unique sequential approach to synthesize penta- and hexa-substituted benzene derivatives. The study, published online on January 26, 2015 in Nature Chemistry, reveals the first example of the controlled synthesis of benzene with different arene groups at all six positions ‘at-will’, demonstrating the potential of this method to synthesize useful aromatic materials in a predictable and programmed manner.
Benzene, first discovered in 1825, is a six-membered carbon ring with a hydrogen attached to each carbon. The six hydrogens can be replaced by different substituents, making benzene an extremely versatile building block in many materials including in pharmaceuticals, agrochemicals, plastics and organic electronic devices. Based on Burnside’s counting theorem, the number of possible substituted benzenes (N) from n different substituents is (2n + 2n^2 + 4n^3 + 3n^4 + n^6)/12. For example, with 10 substituents, the number of possible substitution patterns on benzene will be 86,185. Although there are a vast number of possible substituents that could be attached to benzene, many of the functional hexaarylbenzenes (HABs) possess a symmetrical structure. This is due to lack of a general method to access multi-substituted asymmetric benzenes with complete control over the position of installation. Although there have been reports where up to three or four different aryl groups could be selectively installed onto benzene, this new study shows the selective installation of five or six different arene groups on benzene for the first time.
“We had been working on the development of the programmed synthesis of multiply arylated aromatic systems for over 15 years,” says Kenichiro Itami who is one of the leaders of this research. “Our ultimate goal was to solve the synthetic problem of HABs, which has been extremely difficult due to the structural diversity of benzene and the limited number of synthetic methods.”
“The key to access HABs was to use thiophene (a five membered ring containing a sulfur atom) as the starting material,” says Junichiro Yamaguchi who co-led the research. “In 2009, we had achieved the programmed synthesis of thiophene bearing four different aryl groups via C-H activation. We then improved this method to extend it to the synthesis of multi-substituted benzenes.”
“On a substituted thiophene, we conducted a series of metal-catalyzed coupling reactions, followed by cycloaddition to synthesize HAB,” says Suzuki and Segawa, who are the co-authors of this study. “After numerous attempts to find the right reaction conditions, we were finally able to obtain the crystal structure of a propeller-shaped, radially extended HAB with six different substituents.”
Itami and Yamaguchi’s programmed synthesis has enabled the synthesis of HABs bearing five or six different substituents for the first time. Analysis of these novel unsymmetrical compounds revealed that the otherwise non-fluorescent hexaphenylbenzene could actually be made fluorescent by tuning the substituents on the exterior. These results indicate the future application of this method towards generating new molecules for molecular electronics, nanotechnology and bio-imaging.
“Programmed synthesis of HABs has long been a unresolved problem. Although the yields of our synthesis still needs to be improved, we believe that this methodology will lead to maximizing the structural diversity of benzene derivatives in a programmable fashion, which will lead to understanding structure-property relationships and help discover new functional material,” say Itami and Yamaguchi.